Describe What Happens in Alpha Decay.
In this decay the atomic number of the atom. What happens in beta decay.
Name an element that goes through this kind of decay.
. Write a description of what happens in the beta decay of an atom. This is alpha decay of lawrencium an unstable synthetic element where it decays into mendelevium- 251 and emits out an alpha particle. 19 Describe what changes occur during alpha decay.
There are three major types of radioactive decay. In alpha decay the mass number the total number of protons and neutrons of the parent nuclide decreases by. A negatively charged ion as one attracted to the anode in electrolysis.
1jaiz4 and 1 more users found this answer helpful. Neutron splits into a proton and a beta particle. Alpha decay is one of the nuclear decay process.
A The mass number and atomic number decreases. Use Reset Nucleus to watch the process repeatedly. What is ionizing radiation and why is it dangerous.
Check your ideas with the Custom atom and reflect on your ideas. Write a description of what happens in the alpha decay. Student directions Alpha Decay Names_____ Directions.
Investigating ³Alpha Decay a. The mass number decreases by 4. Alpha decay beta decay and gamma decay.
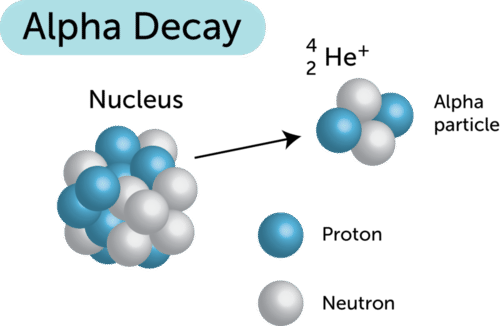
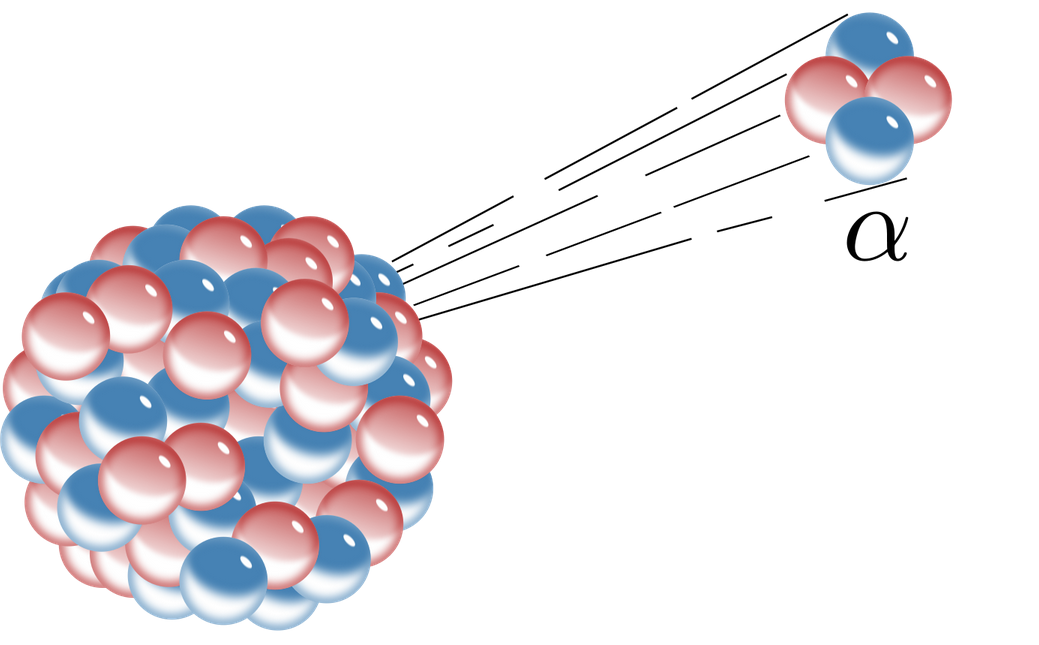
Radioactive decay With the wrong number of neutrons nuclei can fall apart. An alpha particle is identical to the nucleus of a helium-4 atom which consists of two protons and two. X Explain alpha decay process.
A The mass number and atomic number decreases. Describe what happens in alpha decay. In alpha decay an alpha particle a helium nucleus is emitted from the radioactive atom and the atom therefore loses 2 protons and becomes a new element.
We know that alpha particles are represented by a Helium atom ie. Start on the Single Atom tab - observe the decay of Hydrogen-3 and Carbon- 14. Use Reset Nucleus to watch the process repeatedly.
So after the alpha decay the element cardinalium-100 will produce an element wh View the full answer. What happens in alpha decay. Asked Sep 6 2019 in Physics Space Science by mrrandom777.
The nucleus has its atomic number reduced by 2 and its mass number is reduced by 4 2 protons and 2 neutrons are removed. Name two elements that go through alpha decay. In this decay a heavier nuclei is converted to lighter nuclei with the release of alpha particle.
Start on the Single Atom tab - observe the decay of Polonium -211. Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle helium nucleus and thereby transforms or decays into a different atomic nucleus with a mass number that is reduced by four and an atomic number that is reduced by two. In this decay process alpha particle is released.
Atomic goes down by two mass goes down by four. Open Beta Decay 3. Alpha Decay In Alpha Decay the nucleus is split into 2 parts with one of these parts the alpha particle zooming off into space.
A radioactive process in which an alpha particle is emitted from the nucleus of an atom decreasing its atomic number by two. As the alpha particle leaves the atom taking protons and neutrons with it the atomic number changes and. In the case of alpha decay the particle consists of two protons and two neutrons tightly bound together.
The atomic number decreases by 2. This particle happens to be identical in structure to the nucleus of a helium atom and much of the Earths supply of helium actually comes from alpha decay. When the element cardinalium-100 undergoes the following radioactive decays such as.
Open Alpha Decay 1. Beta Decay In Beta Decay minus a neutron turns into a proton. Protons are positively charged.
In beta decay an atom emits out an electron with an antineutrino or a. Alpha decay involves the loss of a helium nucleus beta decay concerns protons turning into neutrons or vice versa and gamma decay involves the emission of energy without changing the original atom. An alpha particle is also a Helium-4 nucleus so it is written as _24textrmHe and is also sometimes written as _24alpha.
What happens during beta decay. Nucleus loses 2 protons and 2 neutrons. X Explain what half-life means in terms of single particles and larger samples.
Beta Decay is a type of radioactive decay in which a proton is transformed into a neutron or vice versa inside the nucleus of the radioactive sample. Processes like beta decay and alpha decay allow the nucleus of the radioactive sample to get as close as possible to the optimum neutron proton ratio. Alpha decay type of radioactive disintegration in which some unstable atomic nuclei dissipate excess energy by spontaneously ejecting an alpha particle.
B The mass number and atomic number increases. The alpha particle is also known as helium atom. Because alpha particles have two positive charges and a mass of four units their emission from nuclei produces daughter nuclei having a positive nuclear charge or atomic number two units less than their parents and a.
Alpha decay or α-decay is a type of radioactive decay in which the atomic nucleus emits an alpha particle thereby transforming or decaying into a new atomic nucleus. When a nucleus emits an alpha particle these changes happen. Here the atomic mass number of the newly formed atom will be reduced by four and the atomic number will be reduced by two.
Investigating Beta Decay a. A nucleus will regain stability by emitting alpha or beta particles and then cool down by emitting gamma radiation. While doing so the nucleus emits a beta.
Explain how gamma rays are produced. Asked Sep 5 2019 in Physics Space Science by Gibbz. Alpha particle released carries a mass of 4 units and a charge of 2 units.
Effects of alpha decay. Alpha decay causes the mass number.


Comments
Post a Comment